Therapies
The Sanofi Rare Blood Disorders portfolio of products
At Sanofi, we’re proud of what we’ve built. But we’re always looking for the next innovation in rare blood disorders. It’s a passion for progress that unites our portfolio of trusted brands.
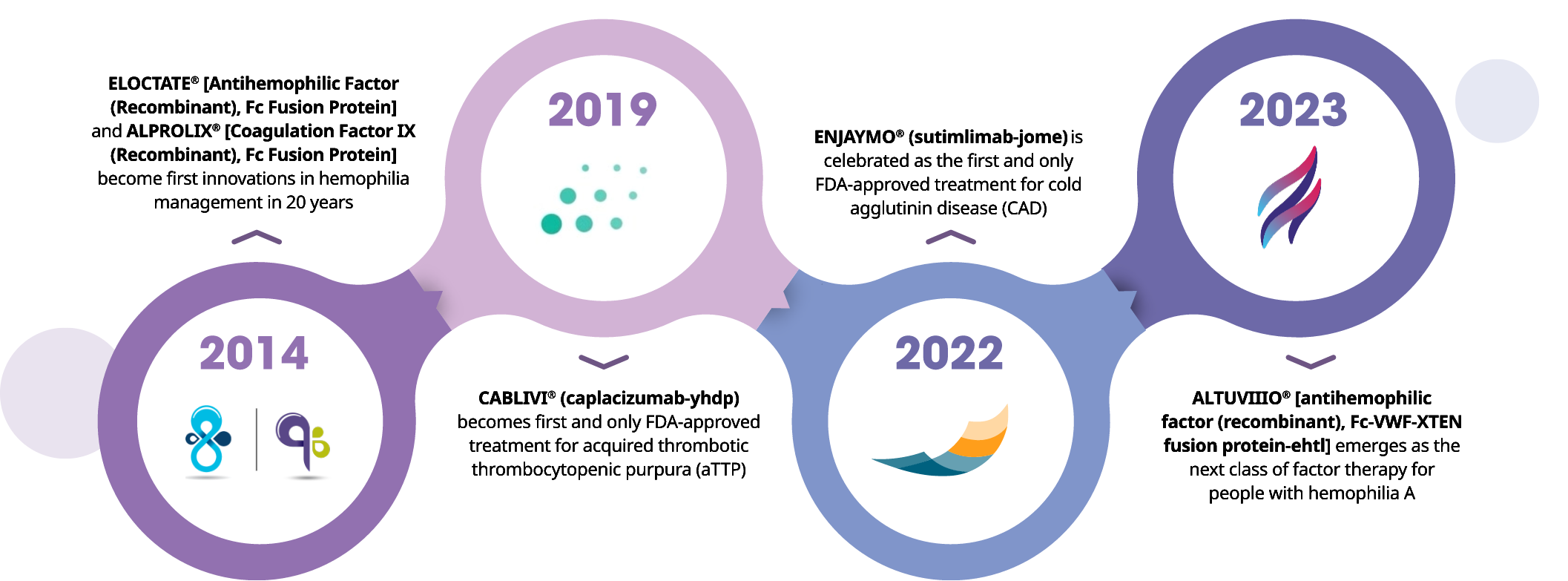
Sanofi Rare Blood Disorders Milestones
Learn more about Sanofi Rare Blood Disorders therapies
![ALTUVIIIO® [antihemophilic factor (recombinant), Fc-VWF-XTEN fusion protein-ehtl] Logo](/_nuxt/img/Altuviiio_R_Logo_RGB.25b5a81.png)
INDICATION
- Routine prophylaxis to reduce the frequency of bleeding episodes
- On-demand treatment & control of bleeding episodes
- Perioperative management of bleeding
ALTUVIIIO is not indicated for the treatment of von Willebrand disease.
![ALPROLIX® [Coagulation Factor IX (Recombinant), Fc Fusion Protein] Logo](/_nuxt/img/alprolix.18cf333.jpg)
INDICATION
- On-demand treatment and control of bleeding episodes
- Perioperative management of bleeding
- Routine prophylaxis to reduce the frequency of bleeding episodes
ALPROLIX is not indicated for induction of immune tolerance in patients with hemophilia B.

INDICATION
![ELOCTATE® [Antihemophilic Factor (Recombinant), Fc Fusion Protein] Logo](/_nuxt/img/eloctate.13a74c6.jpg)
INDICATION
Limitation of Use
ELOCTATE is not indicated for the treatment of von Willebrand disease.

INDICATION


ALTUVIIIO Important Safety Information and Indication
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS:
ALTUVIIIO is contraindicated in patients who have had severe hypersensitivity reactions, including anaphylaxis, to the product or its excipients.
WARNINGS AND PRECAUTIONS:
Allergic-type hypersensitivity reactions, including anaphylaxis, may occur with ALTUVIIIO. Allergic-type hypersensitivity reactions were not reported in the clinical trials. Advise patients to discontinue use of ALTUVIIIO if hypersensitivity symptoms occur and contact a physician and/or seek immediate emergency care.
Formation of neutralizing antibodies (inhibitors) to Factor VIII are possible following administration of ALTUVIIIO. Neutralizing antibodies were not reported in the clinical trials. Monitor all patients for the development of Factor VIII inhibitors by appropriate clinical observations and laboratory tests.
If assessment of plasma Factor VIII activity is needed, it is recommended to use a validated one-stage clotting assay. The ALTUVIIIO Factor VIII activity level is overestimated by the chromogenic assay and a specific ellagic acid-based aPTT reagent in one-stage clotting assay by approximately 2.5-fold. If these assays are used, divide the result by 2.5 to approximate the patient’s ALTUVIIIO Factor VIII activity level.
ADVERSE REACTIONS:
The most common adverse reactions (>10% of subjects) reported in clinical trials were headache and arthralgia.
INDICATION
ALTUVIIIO® [antihemophilic factor (recombinant), Fc-VWF-XTEN fusion protein-ehtl] is a von Willebrand Factor (VWF) independent recombinant DNA-derived, Factor VIII concentrate indicated for use in adults and children with hemophilia A (congenital factor VIII deficiency) for:
Routine prophylaxis to reduce the frequency of bleeding episodes
On-demand treatment & control of bleeding episodes
Perioperative management of bleeding
Limitation of Use:
ALTUVIIIO is not indicated for the treatment of von Willebrand disease.
Please see Full Prescribing Information
ALPROLIX Important Safety Information and Indication
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS:ALPROLIX is contraindicated in patients who have a known history of hypersensitivity reactions, including anaphylaxis, to the product or its excipients.
WARNINGS AND PRECAUTIONS:Allergic-type hypersensitivity reactions, including anaphylaxis, are possible with factor replacement therapies, and have been reported with ALPROLIX. Discontinue use of ALPROLIX if hypersensitivity symptoms occur, and initiate appropriate treatment.
Formation of neutralizing antibodies (inhibitors) to Factor IX has been reported following administration of ALPROLIX. Patients using ALPROLIX should be monitored for the development of Factor IX inhibitors. Clotting assays (e.g., one-stage) may be used to confirm that adequate Factor IX levels have been achieved and maintained.
The use of Factor IX products has been associated with the development of thromboembolic complications.
Nephrotic syndrome has been reported following attempted immune tolerance induction in hemophilia B patients with Factor IX inhibitors and a history of allergic reactions to Factor IX. The safety and efficacy of using ALPROLIX for immune tolerance induction have not been established.
ADVERSE REACTIONS:The most common adverse reactions (incidence ≥1%) in previously untreated patients were injection site erythema, hypersensitivity, and Factor IX inhibition. The most common adverse reactions (incidence ≥1%) in previously treated patients were headache, oral paresthesia, and obstructive uropathy.
INDICATION
ALPROLIX® [Coagulation Factor IX (Recombinant), Fc Fusion Protein] is a recombinant DNA derived, coagulation Factor IX concentrate indicated in adults and children with hemophilia B for:
On-demand treatment and control of bleeding episodes
Perioperative management of bleeding
Routine prophylaxis to reduce the frequency of bleeding episodes
Limitation of Use:
ALPROLIX is not indicated for induction of immune tolerance in patients with hemophilia B.
Please see Full Prescribing Information
CABLIVI Important Safety Information and Indication
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS:CABLIVI is contraindicated in patients with a previous severe hypersensitivity reaction to caplacizumab-yhdp or to any of its excipients. Hypersensitivity reactions have included urticaria.
WARNINGS AND PRECAUTIONS:
Hemorrhage:
CABLIVI increases the risk of bleeding. In clinical studies, severe bleeding adverse reactions of epistaxis, gingival bleeding, upper gastrointestinal hemorrhage, and metrorrhagia were each reported in 1% of subjects. Overall, bleeding events occurred in approximately 58% of patients on CABLIVI versus 43% of patients on placebo.
In the postmarketing setting cases of life-threatening and fatal bleeding were reported in patients receiving CABLIVI.
The risk of bleeding is increased in patients with underlying coagulopathies (e.g. hemophilia, other coagulation factor deficiencies). It is also increased with concomitant use of CABLIVI with drugs affecting hemostasis and coagulation.
Avoid concomitant use of CABLIVI with antiplatelet agents or anticoagulants. If clinically significant bleeding occurs, interrupt use of CABLIVI. Von Willebrand factor concentrate may be administered to rapidly correct hemostasis. If CABLIVI is restarted, monitor closely for signs of bleeding.
Withhold CABLIVI for 7 days prior to elective surgery, dental procedures or other invasive interventions. If emergency surgery is needed, the use of von Willebrand factor concentrate may be considered to correct hemostasis. After the risk of surgical bleeding has resolved, and CABLIVI is resumed, monitor closely for signs of bleeding.
The most common adverse reactions (>15% of patients) were epistaxis (29%), headache (21%) and gingival bleeding (16%).
CONCOMITANT USE OF ANTICOAGULANTS OR ANTIPLATELET AGENTS:Concomitant use of CABLIVI with any anticoagulant or antiplatelet agent may increase the risk of bleeding. Avoid concomitant use when possible. Assess and monitor closely for bleeding with concomitant use.
PREGNANCY:There are no available data on CABLIVI use in pregnant women to inform a drug associated risk of major birth defects and miscarriage.
Fetal/neonatal adverse reactions: CABLIVI may increase the risk of bleeding in the fetus and neonate. Monitor neonates for bleeding.
Maternal adverse reactions: All patients receiving CABLIVI, including pregnant women, are at risk for bleeding. Pregnant women receiving CABLIVI should be carefully monitored for evidence of excessive bleeding.
CABLIVI (caplacizumab-yhdp) is indicated for the treatment of adult patients with acquired thrombotic thrombocytopenic purpura (aTTP), in combination with plasma exchange and immunosuppressive therapy.
Please see Full Prescribing Information
ELOCTATE Important Safety Information and Indication
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS:
ELOCTATE is contraindicated in patients who have had life-threatening hypersensitivity reactions to ELOCTATE or its excipients.
WARNINGS AND PRECAUTIONS:Hypersensitivity reactions have been reported with ELOCTATE. Allergic-type hypersensitivity reactions, including anaphylaxis, have been reported with Factor VIII replacement products. Immediately discontinue ELOCTATE and initiate appropriate treatment if hypersensitivity reactions occur.
Formation of neutralizing antibodies (inhibitors) to Factor VIII has been reported following administration of ELOCTATE. Patients using ELOCTATE should be monitored for the development of Factor VIII inhibitors. Clotting assays (e.g., one-stage) may be used to confirm that adequate Factor VIII levels have been achieved and maintained.
Hemophilic patients with cardiovascular risk factors or diseases may be at the same risk to develop cardiovascular events as non-hemophilic patients when clotting has been normalized by treatment with Factor VIII.
If a central venous access device (CVAD) is required, risk of CVAD-related complications including local infections, bacteremia, and catheter-site thrombosis should be considered.
The most frequently occurring adverse reactions (incidence >0.5% of subjects) reported in previously treated patients (PTPs) clinical trials were arthralgia, malaise, myalgia, headache, and rash. The most frequently occurring adverse reactions (incidence ≥1.0% of subjects) reported in previously untreated patients (PUPs) clinical trials were Factor VIII inhibition, device-related thrombosis, and rash papular.
INDICATION
ELOCTATE® [Antihemophilic Factor (Recombinant), Fc Fusion Protein] is a recombinant DNA derived, antihemophilic factor indicated in adults and children with Hemophilia A (congenital Factor VIII deficiency) for: on-demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes.
Limitation of Use:
ELOCTATE is not indicated for the treatment of von Willebrand disease.
Please see Full Prescribing Information
ENJAYMO Important Safety Information and Indication
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS:
ENJAYMO is contraindicated in patients with known hypersensitivity to sutimlimab-jome or any of the inactive ingredients.
WARNINGS AND PRECAUTIONS:
Serious Infections Including Those Caused by Encapsulated Bacteria:
- ENJAYMO, a proximal classical complement C1s inhibitor, increases susceptibility to serious infections, including infections caused by encapsulated bacteria e.g. Neisseria meningitidis (any serogroup, including non-groupable strains), Streptococcus pneumoniae, and Haemophilus influenzae type B.
- Life-threatening and fatal infections with encapsulated bacteria have occurred in both vaccinated and unvaccinated patients treated with complement inhibitors.
- Serious infections (bacterial and viral) were reported in 15% (10/66) of patients receiving ENJAYMO in the two phase 3 trials. These infections included urinary tract infection with sepsis, respiratory tract infection, pneumonia, otomastoiditis, and skin infections. One patient (1.5%) died due to Klebsiella pneumoniae.
- Complete or update vaccination against encapsulated bacteria at least 2 weeks prior to administration of the first dose of ENJAYMO, according to the most current ACIP recommendations for patients receiving a complement inhibitor.
- If urgent ENJAYMO therapy is indicated in a patient who is not up to date on their vaccine(s), administer as soon as possible.
- Vaccination does not eliminate the risk of serious encapsulated bacterial infections. Closely monitor patients for early signs and symptoms of serious infection and evaluate patients immediately if an infection is suspected.
-
If ENJAYMO treatment is administered to patients with active
systemic infections, monitor closely for signs and symptoms of
worsening infection. Some infections may become rapidly
life-threatening or fatal if not recognized and treated promptly.
Inform patients of these signs and symptoms and steps to be taken to
seek immediate medical care.
- Consider interruption of ENJAYMO treatment in patients who are undergoing treatment for serious infection.
- Consider patients’ immune status when initiating treatment with ENJAYMO.
Infusion-Related Reactions:
- Administration of ENJAYMO may result in infusion-related reactions. In the two phase 3 trials, 29% (19/66) of patients treated with ENJAYMO experienced infusion-related reactions. One patient permanently discontinued ENJAYMO due to an infusion-related reaction.
- Monitor patients for infusion-related reactions and interrupt if a reaction occurs.
- Discontinue ENJAYMO infusion and institute appropriate supportive measures if signs of hypersensitivity reactions, such as cardiovascular instability or respiratory compromise, occur.
Risk of Autoimmune Disease:
- Based on its mechanism of action, ENJAYMO may potentially increase the risk for developing autoimmune diseases such as systemic lupus erythematosus (SLE). Development of SLE has been associated with inherited classical complement deficiency.
- In clinical trials, 4.5% (3/66) of patients developed a relapse or worsening of previously diagnosed autoimmune disease.
- Monitor ENJAYMO patients for signs and symptoms and manage medically.
Recurrent Hemolysis After ENJAYMO Discontinuation:
- If treatment with ENJAYMO is interrupted, closely monitor patients for signs and symptoms of recurrent hemolysis, eg, elevated levels of total bilirubin or lactate dehydrogenase (LDH) accompanied by a decrease in hemoglobin, or reappearance of symptoms such as fatigue, dyspnea, palpitations, or hemoglobinuria. Consider restarting ENJAYMO if signs and symptoms of hemolysis occur after discontinuation.
ADVERSE REACTIONS:
- The most common adverse reactions in the CADENZA trial (Part A) (incidence ≥18%) are rhinitis, headache, hypertension, acrocyanosis, and Raynaud’s phenomenon. The most common adverse reactions in the CARDINAL trial (incidence ≥25%) are urinary tract infection, respiratory tract infection, bacterial infection, dizziness, fatigue, peripheral edema, arthralgia, cough, hypertension, and nausea.
ELOCTATE
ALPROLIX
CABLIVI
ENJAYMO
ALTIVIIO
INDICATION
ENJAYMO® (sutimlimab-jome) is indicated for the treatment of hemolysis in adults with cold agglutinin disease (CAD).
Please see Full Prescribing Information
ALTUVIIIO Important Safety Information and Indication
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS:
ALTUVIIIO is contraindicated in patients who have had severe hypersensitivity reactions, including anaphylaxis, to the product or its excipients.
WARNINGS AND PRECAUTIONS:
Allergic-type hypersensitivity reactions, including anaphylaxis, may occur with ALTUVIIIO. Allergic-type hypersensitivity reactions were not reported in the clinical trials. Advise patients to discontinue use of ALTUVIIIO if hypersensitivity symptoms occur and contact a physician and/or seek immediate emergency care.
Formation of neutralizing antibodies (inhibitors) to Factor VIII are possible following administration of ALTUVIIIO. Neutralizing antibodies were not reported in the clinical trials. Monitor all patients for the development of Factor VIII inhibitors by appropriate clinical observations and laboratory tests.
If assessment of plasma Factor VIII activity is needed, it is recommended to use a validated one-stage clotting assay. The ALTUVIIIO Factor VIII activity level is overestimated by the chromogenic assay and a specific ellagic acid-based aPTT reagent in one-stage clotting assay by approximately 2.5-fold. If these assays are used, divide the result by 2.5 to approximate the patient’s ALTUVIIIO Factor VIII activity level.
ADVERSE REACTIONS:
The most common adverse reactions (>10% of subjects) reported in clinical trials were headache and arthralgia.
INDICATION
ALTUVIIIO® [antihemophilic factor (recombinant), Fc-VWF-XTEN fusion protein-ehtl] is a von Willebrand Factor (VWF) independent recombinant DNA-derived, Factor VIII concentrate indicated for use in adults and children with hemophilia A (congenital factor VIII deficiency) for:
Routine prophylaxis to reduce the frequency of bleeding episodes
On-demand treatment & control of bleeding episodes
Perioperative management of bleeding
Limitation of Use:
ALTUVIIIO is not indicated for the treatment of von Willebrand disease.
Please see Full Prescribing Information
ALPROLIX Important Safety Information and Indication
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS:ALPROLIX is contraindicated in patients who have a known history of hypersensitivity reactions, including anaphylaxis, to the product or its excipients.
WARNINGS AND PRECAUTIONS:Allergic-type hypersensitivity reactions, including anaphylaxis, are possible with factor replacement therapies, and have been reported with ALPROLIX. Discontinue use of ALPROLIX if hypersensitivity symptoms occur, and initiate appropriate treatment.
Formation of neutralizing antibodies (inhibitors) to Factor IX has been reported following administration of ALPROLIX. Patients using ALPROLIX should be monitored for the development of Factor IX inhibitors. Clotting assays (e.g., one-stage) may be used to confirm that adequate Factor IX levels have been achieved and maintained.
The use of Factor IX products has been associated with the development of thromboembolic complications.
Nephrotic syndrome has been reported following attempted immune tolerance induction in hemophilia B patients with Factor IX inhibitors and a history of allergic reactions to Factor IX. The safety and efficacy of using ALPROLIX for immune tolerance induction have not been established.
ADVERSE REACTIONS:The most common adverse reactions (incidence ≥1%) in previously untreated patients were injection site erythema, hypersensitivity, and Factor IX inhibition. The most common adverse reactions (incidence ≥1%) in previously treated patients were headache, oral paresthesia, and obstructive uropathy.
INDICATION
ALPROLIX® [Coagulation Factor IX (Recombinant), Fc Fusion Protein] is a recombinant DNA derived, coagulation Factor IX concentrate indicated in adults and children with hemophilia B for:
On-demand treatment and control of bleeding episodes
Perioperative management of bleeding
Routine prophylaxis to reduce the frequency of bleeding episodes
Limitation of Use:
ALPROLIX is not indicated for induction of immune tolerance in patients with hemophilia B.
Please see Full Prescribing Information
CABLIVI Important Safety Information and Indication
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS:CABLIVI is contraindicated in patients with a previous severe hypersensitivity reaction to caplacizumab-yhdp or to any of its excipients. Hypersensitivity reactions have included urticaria.
WARNINGS AND PRECAUTIONS:
Hemorrhage:
CABLIVI increases the risk of bleeding. In clinical studies, severe bleeding adverse reactions of epistaxis, gingival bleeding, upper gastrointestinal hemorrhage, and metrorrhagia were each reported in 1% of subjects. Overall, bleeding events occurred in approximately 58% of patients on CABLIVI versus 43% of patients on placebo.
In the postmarketing setting cases of life-threatening and fatal bleeding were reported in patients receiving CABLIVI.
The risk of bleeding is increased in patients with underlying coagulopathies (e.g. hemophilia, other coagulation factor deficiencies). It is also increased with concomitant use of CABLIVI with drugs affecting hemostasis and coagulation.
Avoid concomitant use of CABLIVI with antiplatelet agents or anticoagulants. If clinically significant bleeding occurs, interrupt use of CABLIVI. Von Willebrand factor concentrate may be administered to rapidly correct hemostasis. If CABLIVI is restarted, monitor closely for signs of bleeding.
Withhold CABLIVI for 7 days prior to elective surgery, dental procedures or other invasive interventions. If emergency surgery is needed, the use of von Willebrand factor concentrate may be considered to correct hemostasis. After the risk of surgical bleeding has resolved, and CABLIVI is resumed, monitor closely for signs of bleeding.
The most common adverse reactions (>15% of patients) were epistaxis (29%), headache (21%) and gingival bleeding (16%).
CONCOMITANT USE OF ANTICOAGULANTS OR ANTIPLATELET AGENTS:Concomitant use of CABLIVI with any anticoagulant or antiplatelet agent may increase the risk of bleeding. Avoid concomitant use when possible. Assess and monitor closely for bleeding with concomitant use.
PREGNANCY:There are no available data on CABLIVI use in pregnant women to inform a drug associated risk of major birth defects and miscarriage.
Fetal/neonatal adverse reactions: CABLIVI may increase the risk of bleeding in the fetus and neonate. Monitor neonates for bleeding.
Maternal adverse reactions: All patients receiving CABLIVI, including pregnant women, are at risk for bleeding. Pregnant women receiving CABLIVI should be carefully monitored for evidence of excessive bleeding.
CABLIVI (caplacizumab-yhdp) is indicated for the treatment of adult patients with acquired thrombotic thrombocytopenic purpura (aTTP), in combination with plasma exchange and immunosuppressive therapy.
Please see Full Prescribing Information
ELOCTATE Important Safety Information and Indication
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS:
ELOCTATE is contraindicated in patients who have had life-threatening hypersensitivity reactions to ELOCTATE or its excipients.
WARNINGS AND PRECAUTIONS:Hypersensitivity reactions have been reported with ELOCTATE. Allergic-type hypersensitivity reactions, including anaphylaxis, have been reported with Factor VIII replacement products. Immediately discontinue ELOCTATE and initiate appropriate treatment if hypersensitivity reactions occur.
Formation of neutralizing antibodies (inhibitors) to Factor VIII has been reported following administration of ELOCTATE. Patients using ELOCTATE should be monitored for the development of Factor VIII inhibitors. Clotting assays (e.g., one-stage) may be used to confirm that adequate Factor VIII levels have been achieved and maintained.
Hemophilic patients with cardiovascular risk factors or diseases may be at the same risk to develop cardiovascular events as non-hemophilic patients when clotting has been normalized by treatment with Factor VIII.
If a central venous access device (CVAD) is required, risk of CVAD-related complications including local infections, bacteremia, and catheter-site thrombosis should be considered.
The most frequently occurring adverse reactions (incidence >0.5% of subjects) reported in previously treated patients (PTPs) clinical trials were arthralgia, malaise, myalgia, headache, and rash. The most frequently occurring adverse reactions (incidence ≥1.0% of subjects) reported in previously untreated patients (PUPs) clinical trials were Factor VIII inhibition, device-related thrombosis, and rash papular.
INDICATION
ELOCTATE® [Antihemophilic Factor (Recombinant), Fc Fusion Protein] is a recombinant DNA derived, antihemophilic factor indicated in adults and children with Hemophilia A (congenital Factor VIII deficiency) for: on-demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes.
Limitation of Use:
ELOCTATE is not indicated for the treatment of von Willebrand disease.
Please see Full Prescribing Information
ENJAYMO Important Safety Information and Indication
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS:
ENJAYMO is contraindicated in patients with known hypersensitivity to sutimlimab-jome or any of the inactive ingredients.
WARNINGS AND PRECAUTIONS:
Serious Infections Including Those Caused by Encapsulated Bacteria:
- ENJAYMO, a proximal classical complement C1s inhibitor, increases susceptibility to serious infections, including infections caused by encapsulated bacteria e.g. Neisseria meningitidis (any serogroup, including non-groupable strains), Streptococcus pneumoniae, and Haemophilus influenzae type B.
- Life-threatening and fatal infections with encapsulated bacteria have occurred in both vaccinated and unvaccinated patients treated with complement inhibitors.
- Serious infections (bacterial and viral) were reported in 15% (10/66) of patients receiving ENJAYMO in the two phase 3 trials. These infections included urinary tract infection with sepsis, respiratory tract infection, pneumonia, otomastoiditis, and skin infections. One patient (1.5%) died due to Klebsiella pneumoniae.
- Complete or update vaccination against encapsulated bacteria at least 2 weeks prior to administration of the first dose of ENJAYMO, according to the most current ACIP recommendations for patients receiving a complement inhibitor.
- If urgent ENJAYMO therapy is indicated in a patient who is not up to date on their vaccine(s), administer as soon as possible.
- Vaccination does not eliminate the risk of serious encapsulated bacterial infections. Closely monitor patients for early signs and symptoms of serious infection and evaluate patients immediately if an infection is suspected.
-
If ENJAYMO treatment is administered to patients with active
systemic infections, monitor closely for signs and symptoms of
worsening infection. Some infections may become rapidly
life-threatening or fatal if not recognized and treated promptly.
Inform patients of these signs and symptoms and steps to be taken to
seek immediate medical care.
- Consider interruption of ENJAYMO treatment in patients who are undergoing treatment for serious infection.
- Consider patients’ immune status when initiating treatment with ENJAYMO.
Infusion-Related Reactions:
- Administration of ENJAYMO may result in infusion-related reactions. In the two phase 3 trials, 29% (19/66) of patients treated with ENJAYMO experienced infusion-related reactions. One patient permanently discontinued ENJAYMO due to an infusion-related reaction.
- Monitor patients for infusion-related reactions and interrupt if a reaction occurs.
- Discontinue ENJAYMO infusion and institute appropriate supportive measures if signs of hypersensitivity reactions, such as cardiovascular instability or respiratory compromise, occur.
Risk of Autoimmune Disease:
- Based on its mechanism of action, ENJAYMO may potentially increase the risk for developing autoimmune diseases such as systemic lupus erythematosus (SLE). Development of SLE has been associated with inherited classical complement deficiency.
- In clinical trials, 4.5% (3/66) of patients developed a relapse or worsening of previously diagnosed autoimmune disease.
- Monitor ENJAYMO patients for signs and symptoms and manage medically.
Recurrent Hemolysis After ENJAYMO Discontinuation:
- If treatment with ENJAYMO is interrupted, closely monitor patients for signs and symptoms of recurrent hemolysis, eg, elevated levels of total bilirubin or lactate dehydrogenase (LDH) accompanied by a decrease in hemoglobin, or reappearance of symptoms such as fatigue, dyspnea, palpitations, or hemoglobinuria. Consider restarting ENJAYMO if signs and symptoms of hemolysis occur after discontinuation.
ADVERSE REACTIONS:
- The most common adverse reactions in the CADENZA trial (Part A) (incidence ≥18%) are rhinitis, headache, hypertension, acrocyanosis, and Raynaud’s phenomenon. The most common adverse reactions in the CARDINAL trial (incidence ≥25%) are urinary tract infection, respiratory tract infection, bacterial infection, dizziness, fatigue, peripheral edema, arthralgia, cough, hypertension, and nausea.
ELOCTATE
ALPROLIX
CABLIVI
ENJAYMO
ALTIVIIO
INDICATION
ENJAYMO® (sutimlimab-jome) is indicated for the treatment of hemolysis in adults with cold agglutinin disease (CAD).
Please see Full Prescribing Information
This site is intended for U.S. Healthcare Professionals only.
© 2023 Genzyme Corporation. All rights reserved.
Sanofi is a registered trademark of Sanofi or an affiliate.
All other trademarks are the property of their respective owners.
MAT-US-2309563-v2.0-03/2024
Important safety information and indication
Important Safety Information
CONTRAINDICATIONS
ALTUVIIIO is contraindicated in patients who have had severe hypersensitivity reactions, including anaphylaxis, to the product or its excipients.
WARNINGS AND PRECAUTIONS
- • Allergic-type hypersensitivity reactions, including anaphylaxis, may occur with ALTUVIIIO. Allergic-type hypersensitivity reactions were not reported in the clinical trials. Advise patients to discontinue use of ALTUVIIIO if hypersensitivity symptoms occur and contact a physician and/or seek immediate emergency care.
- • Formation of neutralizing antibodies (inhibitors) to Factor VIII are possible following administration of ALTUVIIIO. Neutralizing antibodies were not reported in the clinical trials. Monitor all patients for the development of Factor VIII inhibitors by appropriate clinical observations and laboratory tests.
- • If assessment of plasma Factor VIII activity is needed, it is recommended to use a validated one-stage clotting assay. The ALTUVIIIO Factor VIII activity level is overestimated by the chromogenic assay and a specific ellagic acid-based aPTT reagent in one-stage clotting assay by approximately 2.5-fold. If these assays are used, divide the result by 2.5 to approximate the patient's ALTUVIIIO Factor VIII activity level.
ADVERSE REACTIONS
The most common adverse reactions (>10% of subjects) reported in clinical trials were headache and arthralgia.
Indication
ALTUVIIIO®[antihemophilic factor (recombinant), Fc-VWF-XTEN fusion protein-ehtl] is a von Willebrand Factor (VWF) independent recombinant DNA-derived, Factor VIII concentrate indicated for use in adults and children with hemophilia A (congenital factor VIII deficiency) for:
- • Routine prophylaxis to reduce the frequency of bleeding episodes
- • On-demand treatment & control of bleeding episodes
- • Perioperative management of bleeding
Limitation of Use
ALTUVIIIO is not indicated for the treatment of von Willebrand disease.
Please see full Prescribing Information.

© 2023 Genzyme Corporation. All rights reserved. ALTUVIIIO and
Sanofi are trademarks of Sanofi or an affiliate.
MAT-US-2300535-v1.0-03/2023


![ALTUVIIIO® [antihemophilic factor (recombinant), Fc-VWF-XTEN fusion protein-ehtl] Logo](/_nuxt/img/altuviiio-logo.6ba8af0.png)